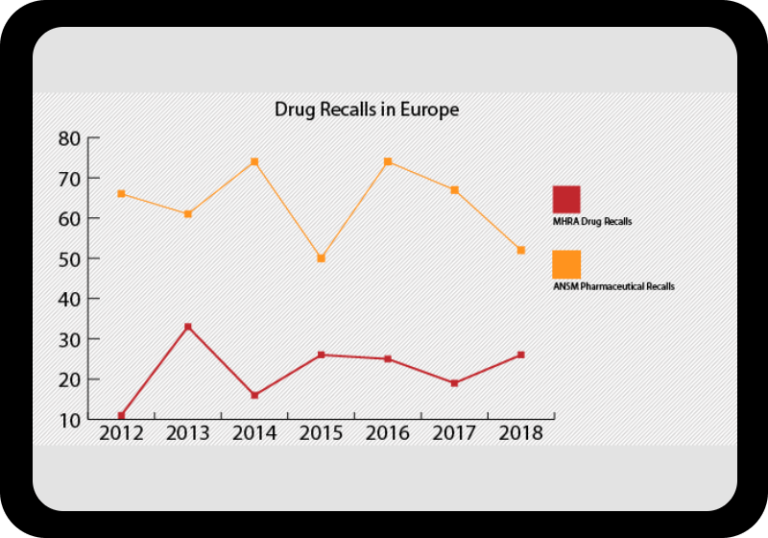
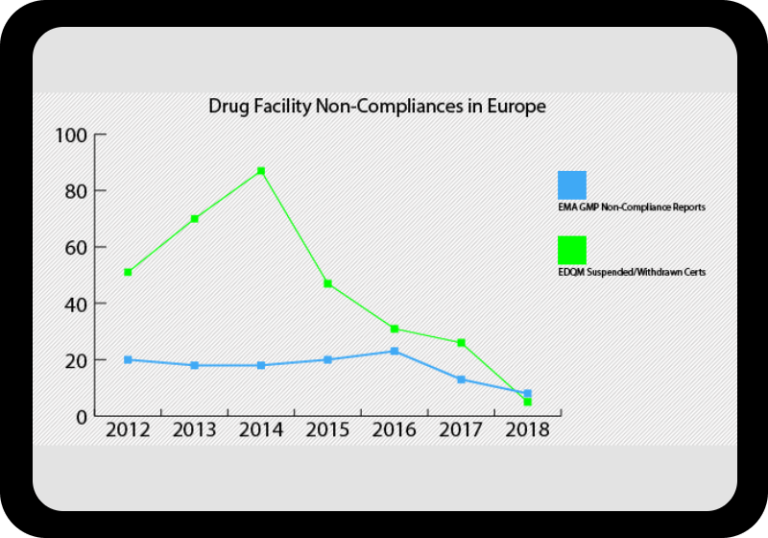
Regulatory Incident Monitor: Pharma Non-Compliance Trending and Alerting
It’s time to move on from complicated, multiple reports. Pharma non-compliance data becomes truly useful when you can generate and view it quickly and easily. Welcome to RIM…
Compliance Reporting Made Easy
Are you looking for a quick and easy way to produce a supplier’s complete non-compliance history? Do you wish there was a way to access multiple data sources without having to check multiple websites?
Let Regulatory Incident Monitor from SCAIR® make your job easier. Use the tool to view any company’s detailed supply chain compliance history. Get full non-compliance data from multiple North American and European medicines regulators. Discover the power of a single report.
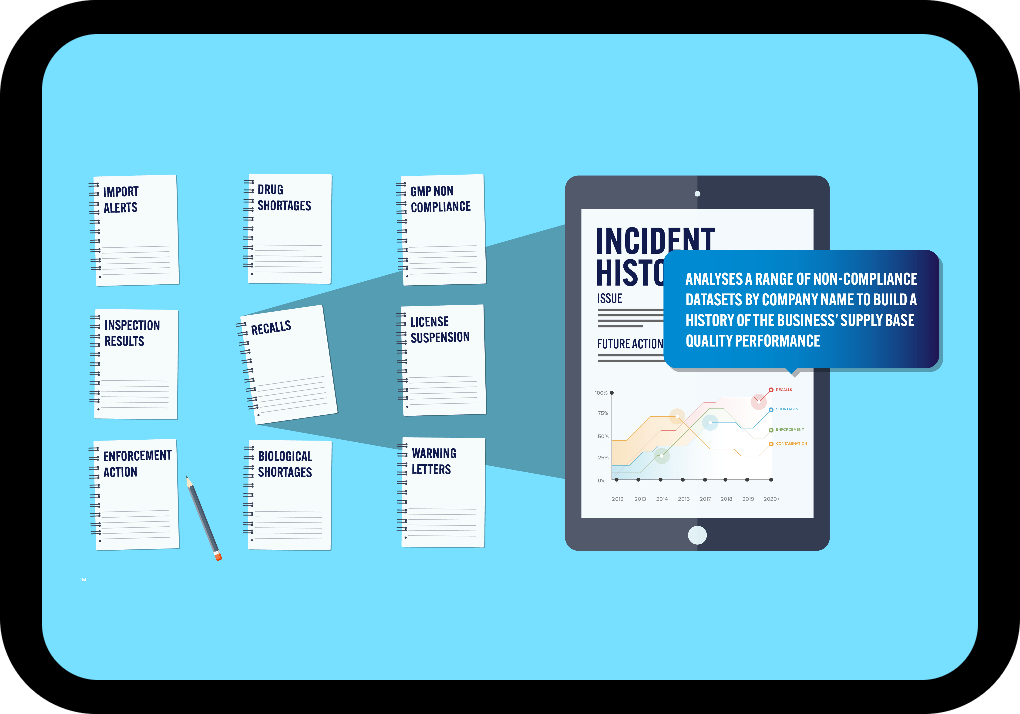
Data from Multiple Regulators
One, powerful searchable database including FDA, Health Canada, EMA, EDQM & MHRA data.
Variants and Subsidiary Names
Powerful search function allows you to include name variations and subsidiary companies.
All Major Non-Compliance Issues
Warning letters, inspection results, GMP non-compliance reports, import alerts, drug shortages, product recalls and more.
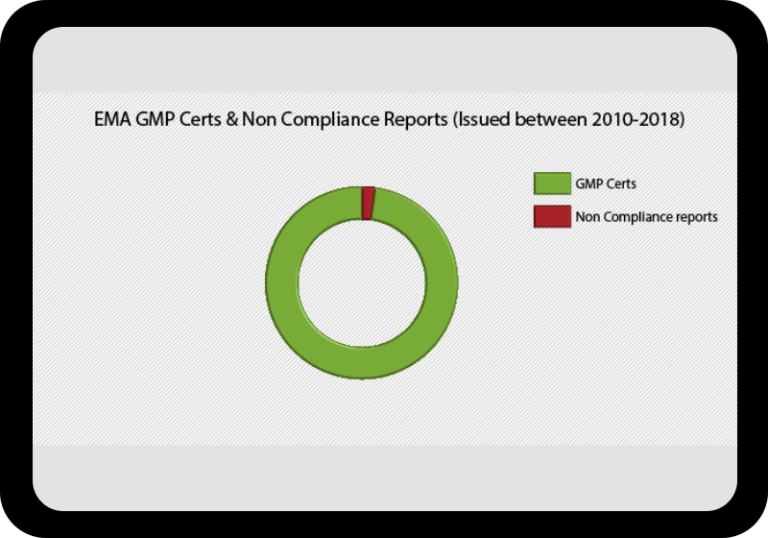
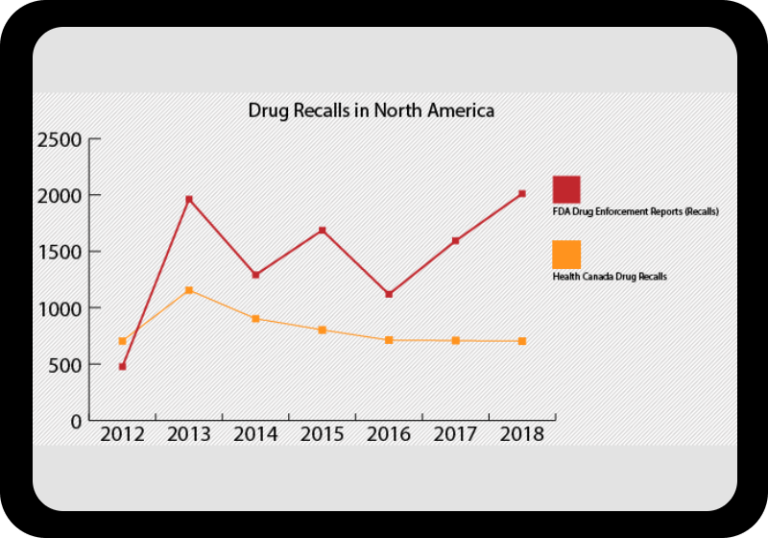
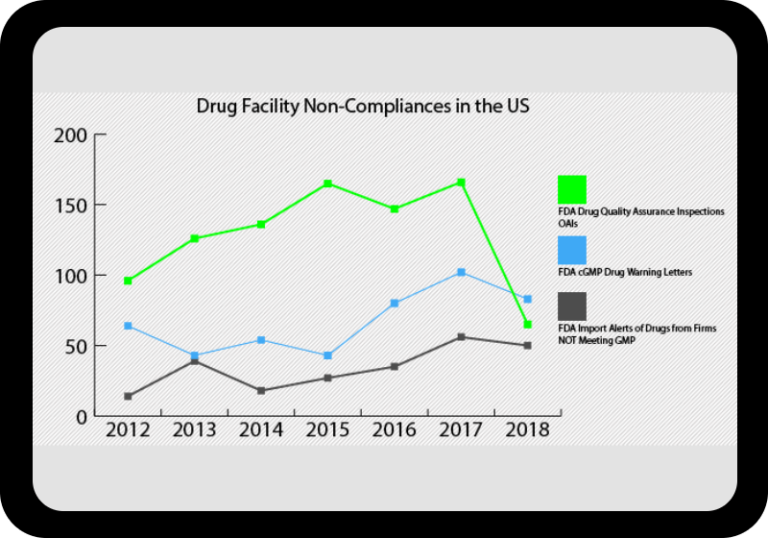
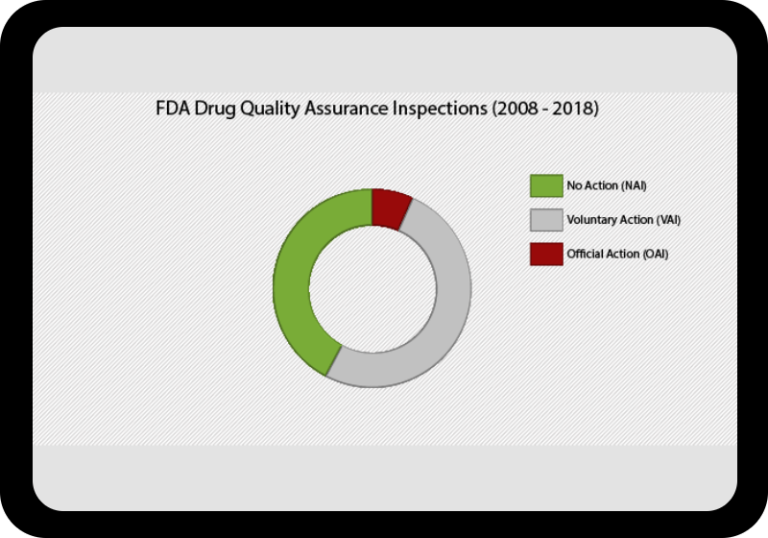
At a Glance Regulatory Trends from FDA and EMA
Browse this wide-ranging dataset to find the North American or European compliance history for your chosen company.
Watch the video to see how it works
How Can Regulatory Incident Monitor Make Your Job Easier?
Perform supplier/CMO due diligence in one report with the complete history of regulatory non-compliances.
No need to visit multiple websites.
Get the most up-to-date consolidated view of published non-compliances
Monitor’s extensive database of company names (extracted from multiple regulatory sources) means that you can select records associated with Company name variants/subsidiaries, to provide a complete history in one report
Get a company’s non-compliance history in seconds, and all in one report.
Access to much more than just FDA data
Monitor combines non-compliance data with registration/certification data to enable supplier selection and validation.
By reporting on major product recalls, shortages and supply interruptions, Monitor can help you: Identify opportunities to fill gaps in the market and Benchmark against competitors
Who is Regulatory
Incident Monitor for?
Quality Managers
who monitor regulatory information from multiple regulators to ensure the quality of their supply chains.
Supply Chain Managers
who need to qualify a new supplier based on their compliance history, for existing supply chain redesign and new product launch.
Quality Assurance Consultants
who need to understand a company’s full compliance history prior to audit.
Risk Managers or Insurers
who need information to assess the quality of a prospect’s site and suppliers as part of their underwriting processes.
Private Equity Investors/Banks
who want to check out the health of a company’s supply chain before M&A.
Procurement Managers
who want to check out the health of a company’s supply chain before M&A.

Want a free trial? Talk to a supply chain risk expert now.
Talk to a supply chain risk expert now
Download the industry-leading white paper Understanding Risk in Pharmaceutical Supply Chains, produced by ReMediES
A collaboration of leading pharma companies, the University of Cambridge and Intersys (the company behind SCAIR®).
Our blog delves deeper into some of the challenges facing Life Science Supply chains.